(PDF) Complement Inhibition for the Treatment of Myasthenia Gravis

The FDA has approved ravulizumab (Ultomiris; Alexion), a terminal compliment C5 inhibitor, for the treatment of patients with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive. With the decision, ravulizumab becomes the first approved long-acting C5 complement inhibitor for this patient population. 1.
Neuromuscular Junction Myasthenia Gravis

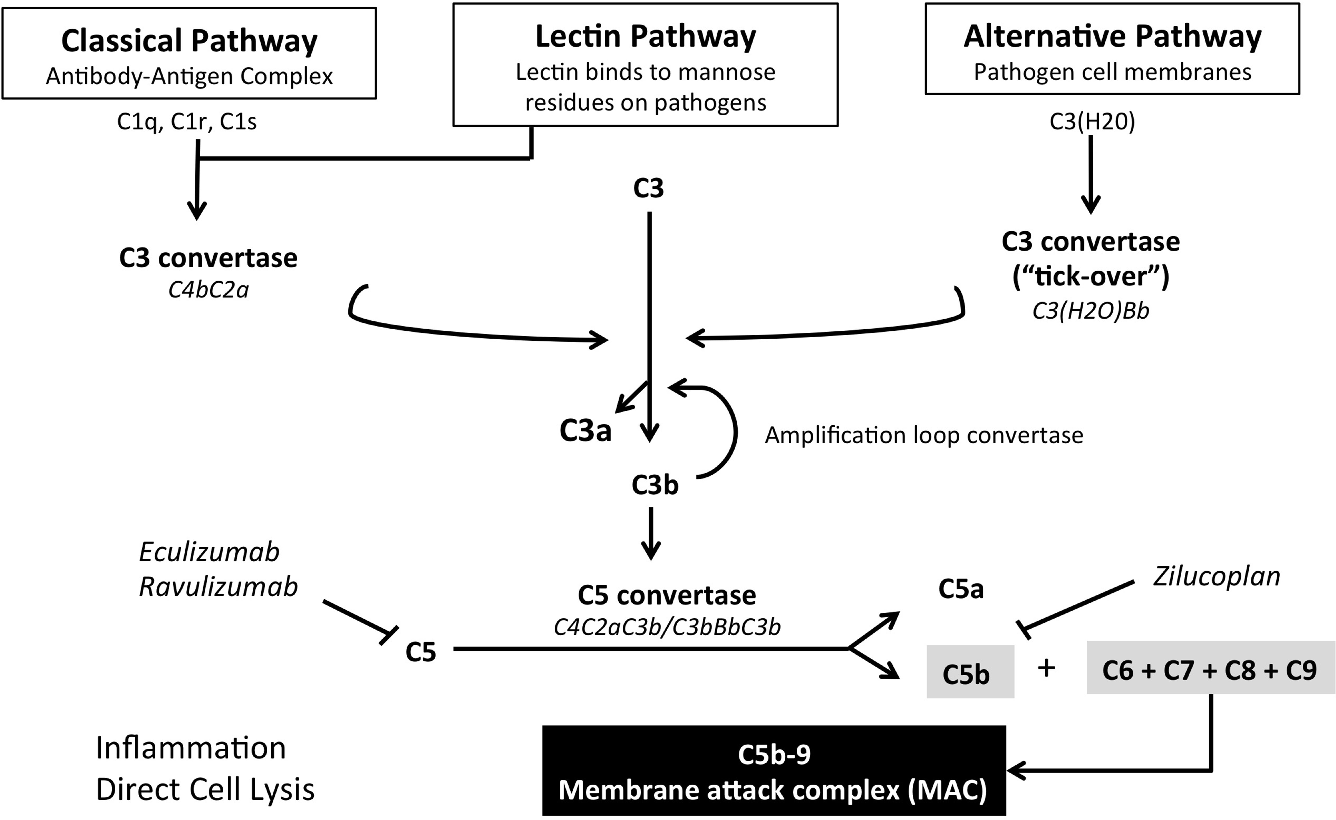
BACKGROUND Generalized myasthenia gravis (gMG) is a rare, chronic, and debilitating autoimmune disease. Activation of the complement system by autoantibodies against the postsynaptic acetylcholine receptor (AChR) leads to destruction of the postsynaptic mem-brane and disruption of neuromuscular transmission. This trial evaluated ravulizumab, a.
Myasthenia gravis the role of complement at the neuromuscular junction Howard 2018 Annals

The randomized placebo-controlled period (RCP) of the phase 3 CHAMPION MG study showed that the complement C5 inhibitor ravulizumab, administered every 8 weeks, provides rapid and sustained symptom improvement in adults with acetylcholine receptor antibody-positive (AChR+) generalized myasthenia gravis (gMG), as determined by both patient.
Ravulizumab for the Treatment of aHUS in Adults Improving Quality of Life Kidney

Ravulizumab is associated with sustained efficacy and safety through 164 weeks for the treatment of acetylcholine receptor antibody-positive (AChRAb+) generalized myasthenia gravis (gMG), according to study results presented at the 2024 American Academy of Neurology (AAN) annual meeting, held from April 13 to 18, 2024, in Denver, Colorado.
Figure 1 from Complement Inhibition for the Treatment of Myasthenia Gravis Semantic Scholar
This article provides a summary of a previously published paper: Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis. The paper reported the results of the CHAMPION-MG trial which investigated the drug ravulizumab in the rare disease, myasthenia gravis. Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis (MP4 594600 KB)
(PDF) Complement Inhibitor Therapy for Myasthenia Gravis

Long-term Efficacy and Safety of Ravulizumab, a Long-acting Terminal Complement Inhibitor, in Adults with Anti-acetylcholine Receptor Antibody-positive Generalized Myasthenia Gravis: Final Results from the Phase 3 CHAMPION MG Open-label Extension (S15.010). Ravulizumab was well tolerated; no meningococcal infections were reported. Conclusions:
Complement associated pathogenic mechanisms in myasthenia gravis. Semantic Scholar
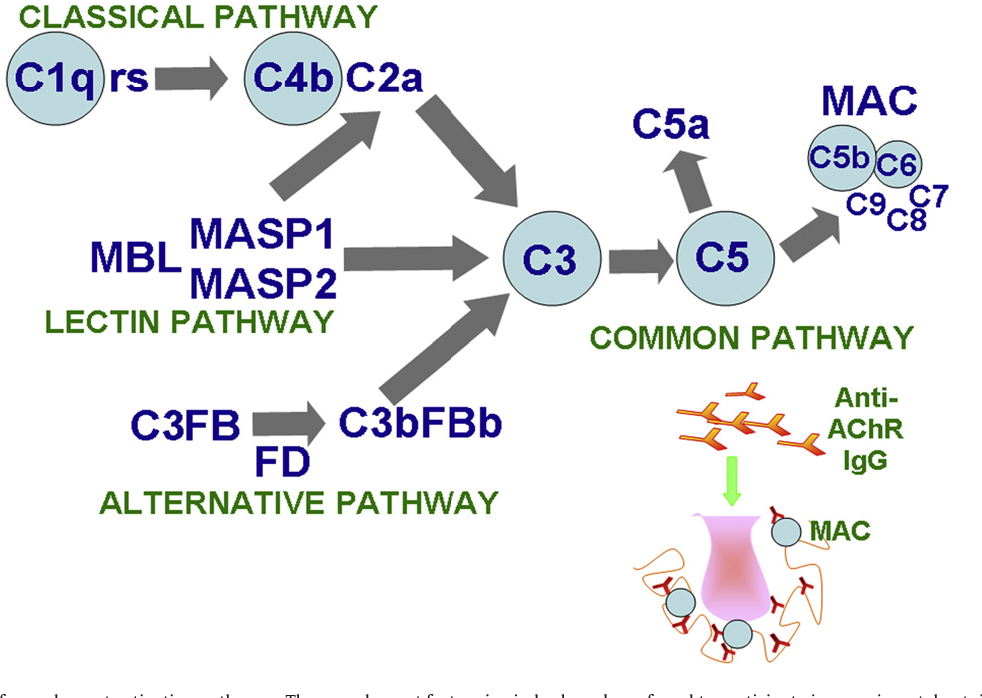
Generalized myasthenia gravis (gMG) is a rare, chronic, and debilitating autoimmune disease. Activation of the complement system by autoantibodies against the postsynaptic acetylcholine receptor (AChR) leads to destruction of the postsynaptic membrane and disruption of neuromuscular transmission. This trial evaluated ravulizumab, a long-acting.
Pathogenic microbes bind the terminal complement pathway inhibitor... Download Scientific Diagram

The terminal complement C5 inhibitor ravulizumab was engineered from the humanized monoclonal antibody eculizumab to have an extended half-life and duration of action.. Mantegazza R, et al. Long-term efficacy and safety of ravulizumab in generalized myasthenia gravis: phase 3 CHAMPION MG study open-label extension. 34th Annual Meeting of the.
45sng Flow Chart Pathophysiology Of Myasthenia Gravis Ppt B09
Howard JF, Vu Thuan, Mantegazza Renato, et al. Long-term efficacy and safety of ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. Neurology. 2022;98:18 Supplement 853.
James Howard, M.D. The Role of Complement in Myasthenia Gravis YouTube

Generalized myasthenia gravis (gMG) is a rare autoimmune disorder affecting the neuromuscular junction (NMJ).. reduction of C3 and C4 levels with increase of complement terminal components in. randomized, double-blind, placebo-controlled multicenter trial to evaluate safety and efficacy of ravulizumab in complement-inhibitor-naïve adult.
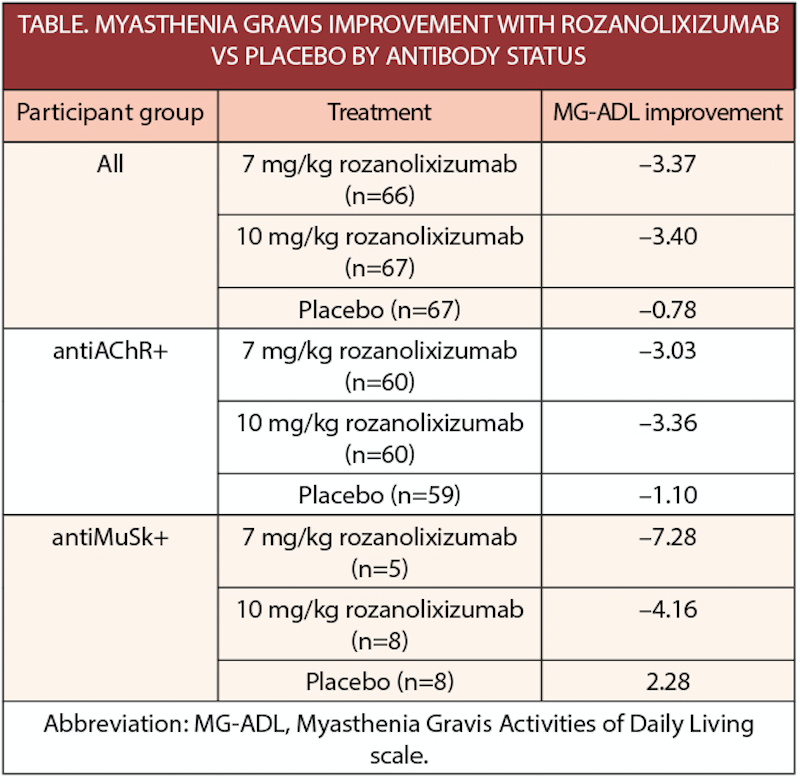
Rozanolixizumab Significantly Improves Generalized Myasthenia Gravis Practical Neurology
The terminal complement C5 inhibitor ravulizumab was engineered from the humanized monoclonal antibody eculizumab to have an extended half-life and duration of action.. Vu T, Meisel A, Mantegazza R, et al. Long-term efficacy and safety of ravulizumab in generalized myasthenia gravis: phase 3 CHAMPION MG study open-label extension. 34th.
(PDF) Summary of Research Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia

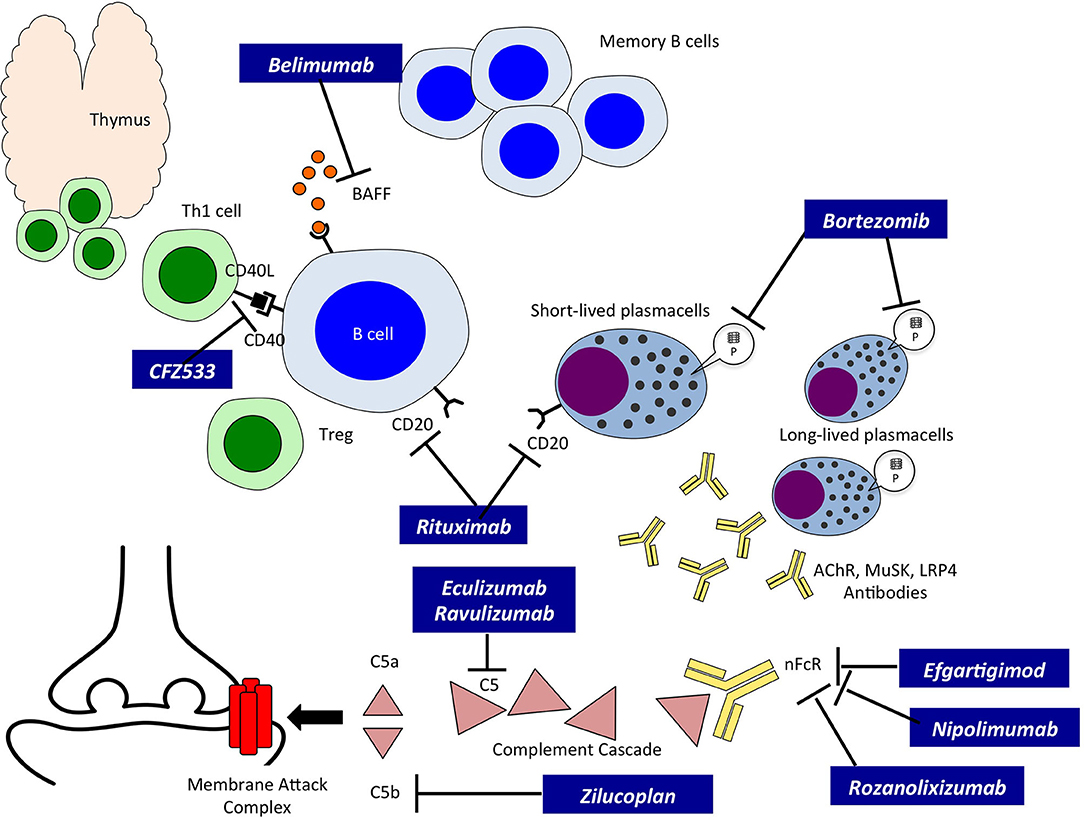
Zilucoplan treatment showed rapid and clinically meaningful improvements in myasthenia gravis-specific efficacy outcomes, had a favourable safety profile, and was well tolerated, with no major safety findings. Zilucoplan is a new potential treatment option for a broad population of patients with AChR-positive generalised myasthenia gravis. The long-term safety and efficacy of zilucoplan is.
NEJM Evidence on Twitter "In this RCT, Ravulizumab provided rapid and efficacious treatment of

This article provides a summary of a previously published paper: Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis. The paper reported the results of the CHAMPION-MG trial which investigated the drug ravulizumab in the rare disease, myasthenia gravis. Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia.
Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis NEJM Evidence

Ravulizumab (ULTOMIRIS ®) is the first long-acting complement C5 inhibitor (administered intravenously every 8 weeks) to be approved in several countries globally, for adults with generalised myasthenia gravis (gMG) who are anti-acetylcholine receptor antibody-positive (AChR Ab+).In the phase III CHAMPION MG trial, intravenous ravulizumab was associated with statistically significant.
Terminal Complement Inhibitor Ravulizumab in Generalized Myasthenia Gravis NEJM Evidence

Efficacy and Safety of Ravulizumab, a Long-acting Terminal Complement Inhibitor, in Adults with Anti-Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: Results from the Phase 3 CHAMPION MG Study (P1-1.Virtual). Ravulizumab is a potent terminal complement C5 inhibitor. Engineered to have a long half-life that permits a.
Mechanism of Myasthenia gravis (MG) caused by immune checkpoint... Download Scientific Diagram

lished paper: Terminal Complement Inhibitor Ravu-lizumab in Generalized Myasthenia Gravis. The paper reported the results of the CHAMPION-MG trial which investigated the drug ravulizumab in the rare disease, myasthenia gravis. Keywords: Anti-AChR antibody; Complement; Monoclonal antibody; Myasthenia gravis; Ravulizumab; Patient-centered outcomes